To find out if your child may be eligible contact a study site location near you.
What is Lennox-Gastaut Syndrome?
Lennox-Gastaut Syndrome (LGS) is a severe epilepsy syndrome that develops in young children and often leads to lifelong disability. Nobody is born with LGS. It develops over time. LGS is a rare disease (approximately one person in every 2,000). About 50,000 people in the United States and 1 million people worldwide have LGS. For more information on LGS, please visit www.lgsfoundation.org.
Tuberous Sclerosis Complex (TSC) is a genetic disorder that causes tumors to form in many different organs, primarily in the brain, eyes, heart, kidney, skin and lungs. The aspects of TSC that most strongly impact quality of life are generally associated with the brain: seizures, developmental delay, intellectual disability and autism. At least two children born each day will have TSC. Current estimates place tuberous sclerosis complex-affected births at one in 6,000. Nearly 1 million people worldwide are estimated to have TSC, with approximately 50,000 in the United States. For more information on TSC, please visit www.tscalliance.org.
What is Dravet Syndrome?
Dravet syndrome is a rare type of epilepsy that starts in the first year of a baby’s life. Its first occurrence is usually a long-lasting seizure (more than five minutes) that’s triggered by a high fever. Children with Dravet syndrome have a wide range of seizure types and severity. They also have many other symptoms, including developmental setbacks, speech and language problems, and balance and walking issues. Researchers estimate that between one in 15,700 and one in 40,000 infants born in the U.S. have Dravet syndrome. For more information, please visit www.dravetfoundation.org.
What do I need to know about the Study?
The Jazz Piccolo Study will involve up to 10 visits to a study clinic and 12 telephone calls and will include a:
- 4 week screening/baseline period
- 52-week treatment period
- 10-day taper following completion of the 52-week treatment regiment
- 4-week safety/follow-up period
Children who are eligible and participate in the Jazz Piccolo Study will receive the investigational drug, as well as study-related visits, tests and assessments, at no cost. Participants can stop taking part in the clinical trial at any time without giving a reason. Caregivers of participants may also be reimbursed for some study-related expenses, such as costs associated with travel and hotels.
The research team will be able to explain more about what the Jazz Piccolo Study will involve, and it is up to you to decide if you want your child to take part. Participation in this study is voluntary. Your decision to participate or not participate will have no effect on the medical care your child receives now or in the future.
Is my Child Eligible to Participate in the Jazz Piccolo Study?
Here is a list of key Inclusion/Exclusion Criteria to participate in the study:
KEY INCLUSION CRITERIA
- Children with confirmed:
- – Tuberous Sclerosis Complex: 1 month to < 2 years of age; or
- – Dravet Syndrome: 1 year to < 2 years of age, or
- – Lennox-Gastaut Syndrome: 1 year to < 2 years of age
- Has seizures not adequately controlled through their current antiseizure medications
- Currently receiving 1 or more antiseizure medications
- A suitable Video EEG or VEEG for confirmation of diagnosis; VEEG is short for a video EEG (electroencephalograph) which records a patient’s experience on video while an EEG test records brainwave function
KEY EXCLUSION CRITERIA
- Clinically significant unstable medical condition other than epilepsy
- Clinically significant symptoms or illness which could affect the seizure frequency
- Has undergone surgery for epilepsy within 6 months
- Significantly impaired hepatic (liver) function
- Currently using recreational or medicinal cannabis, cannabinoid-based medications or CBD
Frequently Asked Questions
What is a study?
Why are studies important?
What is the purpose of the Piccolo study?
What will the Piccolo study involve?
This clinical study involves 3 stages, (1) screening, (2) treatment, and (3) end of treatment.
The first visit will be a visit to the study center. For the second visit, you will receive a telephone call from the study staff. During these visits, the study staff will ask you about your child’s medical history, epilepsy history, demographic data, any medications your child may be taking, and any anti-seizure medication, rescue medication, or vaccination he or she has received.
Some tests will be performed to check that it is appropriate for your child to take part. If the tests show that it is appropriate (known as ‘eligible’), you and your child will be asked to attend up to 9 additional visits at the study site and have up to 11 additional telephone appointments.
Your child may also have a video EEG, which is a test to record the electrical activity of the brain. In certain cases, the video EEG may be collected from your child’s medical record, if it is available.
You will also be asked to complete an electronic daily diary throughout the study to record information on your child’s quality of life, their seizures, any changes in their health, and any other medications he or she may be taking. You will also discuss the seizure types your child is experiencing with the study physician.
If your child is eligible at the beginning of the treatment period, they will be enrolled in the study and be given GWP42003 P (the study medicine). At each visit, the study physician will perform a physical examination, to ensure your child is in good health. Safety tests will be performed, which include measuring your child’s vital signs and collecting blood and urine samples for laboratory safety tests. Your child will have electrocardiograms, which take a tracing of the electrical impulses of their heartbeat.
Extra blood samples at some visits will be taken to test how the study medicine has been absorbed into your child’s body. You will also need to record details of your child’s meals (timing and content) in the electronic diary for 24 hours before each of the visits where certain blood tests known as PK or pharmacokinetics samples are collected.
Your child will also have another video EEG at the End-of-Treatment Visit which will last up to 24 hours depending on how long the investigator feels is necessary, and although unlikely, possibly may require an overnight stay. You will also be asked to use the electronic diary to complete questionnaires on your child’s quality of life.
Will compensation for time and travel be provided?
Caregivers of children who qualify to take part in the study may receive reimbursement for time and travel. Please discuss this with the study team.
Is there a cost to participate?
- Your child will receive study-related care from a team of experienced doctors and nurses throughout the study
- All study-related visits, tests, assessments, and investigational medication will be provided at no cost to you
What else do I need to consider?
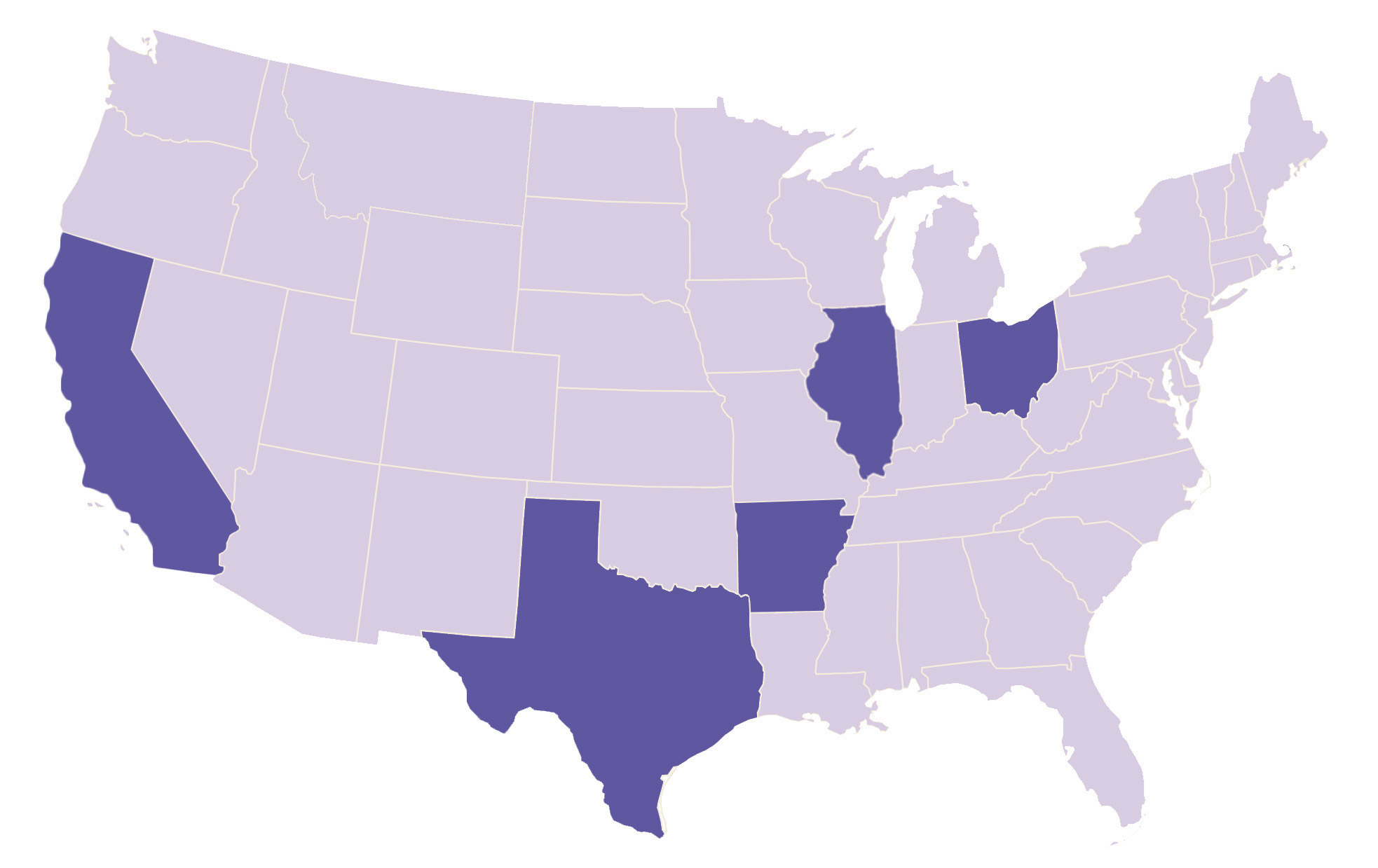
Where are the study sites located?
The Jazz Piccolo Study Is Taking Place Now!
Contact the site closest to you to learn if your child is eligible.
There are Jazz Piccolo study sites located in the United States currently recruiting.
View the study sites by location to determine which is closest to you.
Please check back with us as more sites will be added soon!
Ann & Robert H Lurie Children's Hospital of Chicago
Priyamvada Tatachar
Principal Investigator
Sofia Mirshed
Clinical Study Coordinator
+1 312.227.4525
smirshed@luriechildrens.org
Arkansas Children's Hospital
SITE CONTACTS:
Virginia Willis
Principal Investigator
Ashley Bryan
Clinical Study Coordinator
+1 501.364.3122
bryanAR@archildrens.org
Cincinnati Children's Hospital Medical Center
SITE CONTACTS:
Darcy Krueger
Principal Investigator
Adrienne Victory
Clinical Study Coordinator
Telephone: +1 513-636-8016
Email: Adrienne.Victory@cchmc.org
Baylor College of Medicine/Texas Children's Hospital
SITE CONTACTS:
Anuranjita Nayak
Principal Investigator
Wasiu Olatunji Ahmed
Clinical Research Manager
Telephone: +1 832-824-4126
Email: Wasiu.Ahmed@bcm.edu
David Geffen School of Medicine at UCLA
SITE CONTACTS:
Rajsekar Rajaraman, MD
Principal Investigator
Ruby Escalante
Clinical Research Coordinator
Telephone: +1 310.206.5586
Email: rubyescalante@mednet.ucla.edu
Massachusetts General Hospital
Elizabeth Thiele
Principal Investigator
Carolyn Wilson
Clinical Research Coordinator
Telephone: +1 617-726-0988
Email: cwilson41@mgh.harvard.edu